
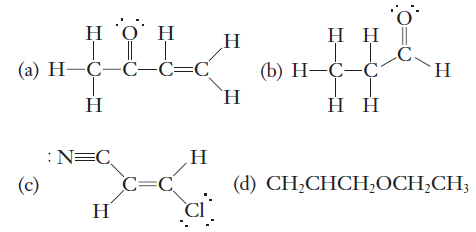
The hydrogen molecule is formed by two hydrogen atoms. This can be understood clearly by taking an example of a hydrogen molecule. S – S Overlapping – s- orbital is spherical in shape and whenever they reach a point of maximum attraction, they overlap and form a sigma bond. Formation of sigma bond is given below between the orbitals-Īs you can see above figure sigma bond is formed by the following three types of overlapping between orbitals – We find sigma bonds in alkanes, alkenes, alkynes. The strongest covalent bond which is formed by the head-on overlapping of the atomic orbitals is called the sigma bond. Thus, in an oxygen molecule, one sigma and one pi bond are formed. While the other set of singly occupied p – orbitals get sideways overlapped and form a pi bond. When two oxygen atoms approach each other to form an oxygen molecule, one set of singly occupied p – orbitals get head-on overlapped axially and form a sigma bond. In the above figure, you can see 2p y, 2p z orbitals of the valence shell of the oxygen atom are singly occupied. The electronic configuration of oxygen atom – By writing its electronic configuration we can see each oxygen atom has two p – orbitals which have only one electron in them in the valence shell. Each oxygen atom has a total of 8 electrons. Oxygen molecules are formed by joining two oxygen atoms covalently. Here you need to note that if only one covalent is present between atoms then it will always be a sigma covalent bond. In general, double covalent bonds consist of one pi and one sigma bond while triple covalent bonds consist of one sigma and two pi – bonds. The molecular orbital of the pi bond is oriented above and below the plane containing the nuclear axis.Īll atoms of the molecule must be in the same plane if the pi bond is formed in the molecule. In pi bonds, the electron density is concentrated in the region perpendicular to the bond axis. Hence, pi bonds are weaker than sigma bonds. In pi bonds, overlapping takes place at the side of the two lobes of p – orbitals so the extent of overlapping is less than the sigma bond. Pi bonds are formed by sideways overlapping of two parallelly oriented pi orbitals of adjacent atoms. The formation of pi bond is given below between the two orbitals The electrons which take part in the formation of pi covalent bonds are called pi – electrons. The covalent bond which is formed by lateral overlapping of the half-filled atomic orbitals (p - orbitals) of atoms is called a pi bond. Here we will discuss pi bonds in detail and will have a short look at sigma bonds and the difference between pi and sigma bonds. Here in this article, we are going to discuss sigma and pi bonds which are covalent bonds only but are formed by different types of overlapping between orbitals.
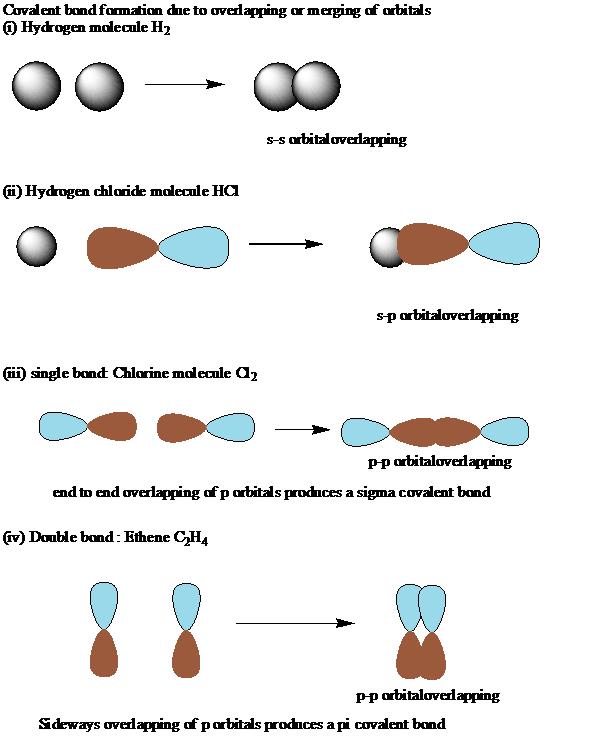
Covalent bonds are those bonds that are formed by sharing of electrons between two atoms. Chemical bonds are classified into covalent bonds, coordinate bonds, ionic bonds and hydrogen bonds.
Chemical bonds are forces that keep atoms joined together.


 0 kommentar(er)
0 kommentar(er)
